DEVELOPMENT OF LIGAND-BLOCKING MONOCLONAL ANTIBODIES
THE CHALLENGE
Purification of membrane proteins is generally tedious and difficult because they are removed from their native membrane en- vironment into a detergent buffer that can only partially mimic the physical and chemical properties of a lipid membrane. Thus, many membrane proteins – multipass transmembrane proteins – do not retain their native conformation after extraction and reconstitution or do so only partially or only under very special buffer conditions. If such structurally compromised proteins are used as antigens for immunization, the probability of obtaining antibodies capable of recognizing the target protein in its native context is very low. In this case study, we discuss the successful development of antibodies against formyl peptide receptor-like 1 (FPRL1), a challenging GPCR protein.
THE SOLUTION
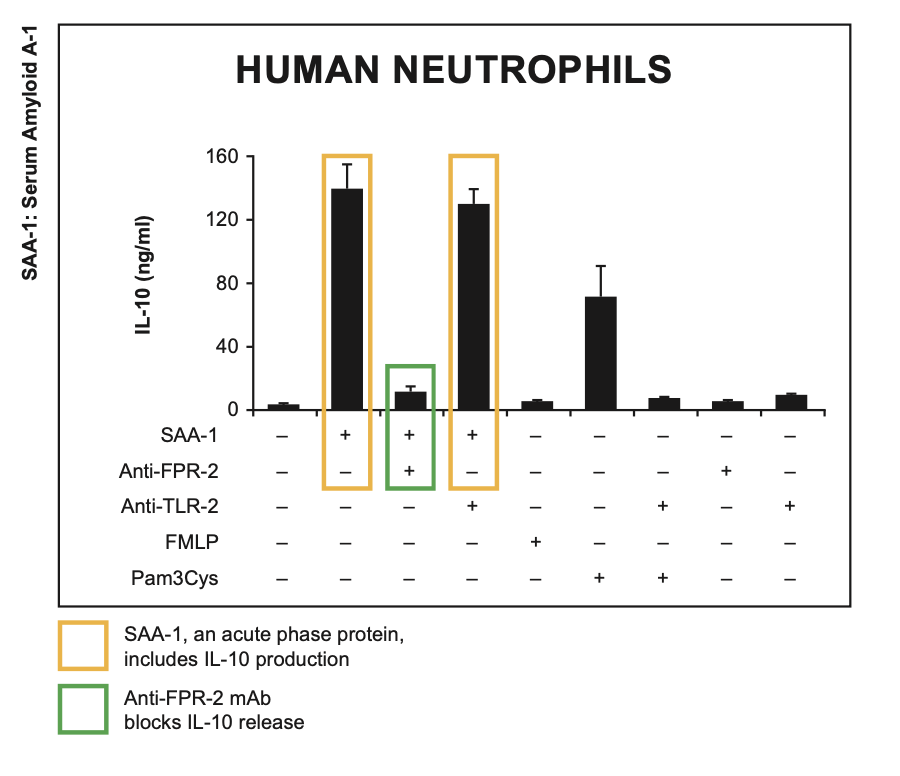
In developing the strategy for producing SAA-1 blocking antibodies, it was important not to disrupt the native conformation of FPRL1 – non-linear epitopes should be preserved. The use of peptides or purified protein fragments was therefore ruled out. We decided to genetically immunize with specially designed immunization constructs that coded for the complete FPRL1 protein.
THE SIGNIFICANCE
We were able to express the protein in vivo and achieve an immune response of the host animal. Subsequent cell fusion yielded dozens of antibodies, some of which had the desired agonistic functionality. Genovac was able to develop antibodies that can be useful therapeutically by decreasing the frequency of immunosuppressive neutrophils and restoring tumor-specific immune responses.
Download full case study as a PDF
"*" indicates required fields